If you could read the mind of someone in pain, it would make management a whole lot easier. Objective biomarkers of pain have been considered a holy grail of pain research, with several groups in the last decades claiming to have discovered pain biomarker candidates from brain activity signatures using neuroimaging techniques like fMRI.
Now, a new paper has identified chronic pain neural biomarkers using electrophysiological recordings of cortical activity. The study, from Prasad Shirvalkar and Edward Chang’s group at University of California San Francisco, USA, is the first ever to identify objective biomarkers of real-world subjective pain intensity over many months from the brain recordings of people living with chronic pain. They found that neural activity patterns in the orbitofrontal cortex (OFC) correlated highly with chronic pain, while acute, experimental pain relied more on anterior cingulate cortex (ACC) activity.
“This is a seminal work that is really going to push the pain field. It’s the first ever chronic brain recordings in patients with chronic pain, up to 180 days. It’s a major technological achievement, as the authors were able to separate acute and chronic pain in different brain regions,” said Paul Geha from the University of Rochester, New York, USA. Geha, an expert in neuroimaging techniques in pain, was not involved in the study.
The study was published 22 May 2023 in Nature Neuroscience.
The challenges of chronic pain biomarkers
“To date, no study has been able to find signals in humans that track actual chronic pain. This is considered a holy grail because there is no way to objectively measure pain; instead, we rely on the simplistic 0-10 scale,” said Shirvalkar, the study’s first author.
Why is this so hard? Shirvalkar explained it’s mostly due to the challenges of finding objective signals in the brain that track subjective experience.
Other groups have used brain imaging techniques like fMRI to find signatures of chronic pain in patients; however, the technique is often skewered by critics who say fMRI only measures a proxy of neuronal activity in the brain (blood oxygen level-dependent, or BOLD, signals), and with poor spatial and temporal acuity.
Another limitation with most older studies, said Shirvalkar, is that they rely on healthy people undergoing pain tasks.
“Actual chronic pain is very different than experimental/acute pain. Even the groups that study actual chronic pain conditions such as low back pain are only able to scan patients’ brains a few times a year. This method cannot be used to evaluate within patient fluctuations of pain severity over minute, day, and month timescales – which are the most relevant and defining characteristic of chronic pain,” he said.
Long-term recordings in chronic pain patients
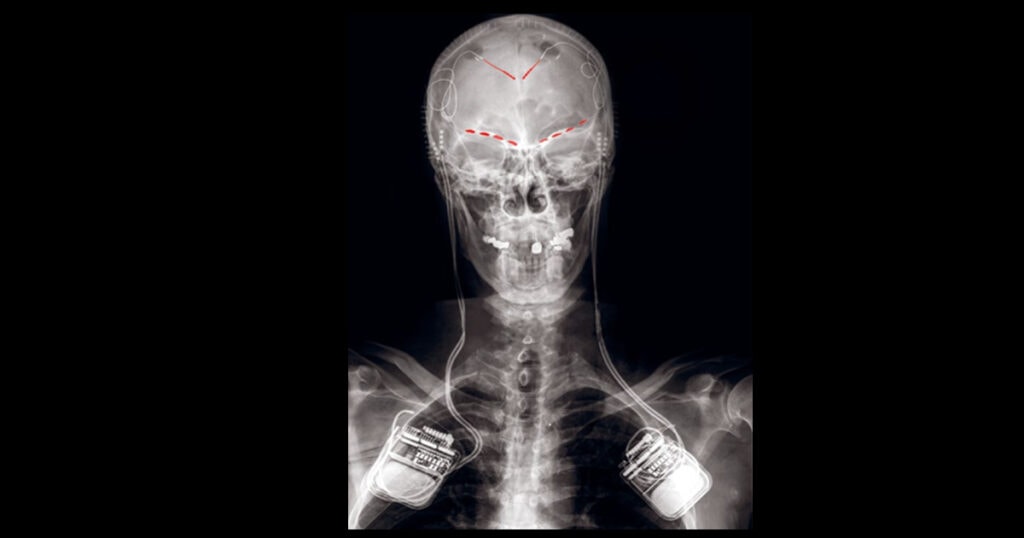
Shirvalkar and his team approached these challenges head on with a new technique: Implanting intracranial electrodes in the brains of patients with chronic pain.
The recordings were performed in four patients, three with post-stroke pain and one with phantom limb pain. The electrodes captured local field potentials (LFP) in the cortex, recording neural activity in groups of neurons with high spatial and temporal resolution.
Perhaps the biggest advantage of this approach is that the recordings can be performed long term, allowing the authors to track brain activity over weeks and months – between 78 and 184 days in this study.
It’s these timepoints the authors analyzed quantitatively, assessing correlations between pain reports and neuronal activity in different cortical regions. In this study, the brain activity was compared to patients’ own subjective pain score reports multiple times per day.
“The localization of neuronal activity is much more accurate than methods like EEG and fMRI. This is a major technological advancement,” said Geha.
Where were the electrodes implanted in the brain? The authors wanted to avoid areas involved in acute somatosensation, like the thalamus and somatosensory cortex, and instead recorded from regions involved with more complex pain processing.
“We decided to study new non-somatosensory related targets for pain relief with brain stimulation and hypothesized that they would harbor important pain biomarkers,” said Shirvalkar.
Here they focused on two regions: The ACC and the OFC – regions implicated in the affective dimension of pain, placebo effects, and expectation related to pain.
Acute pain ACC, chronic pain OFC
The chronic pain “biomarkers” the team found were correlational data between subjective pain scores and neural activity recorded at the same time. They created computational machine learning models that best accurately “predicted” (i.e., tracked) subjective pain states in individual patients based on neural data in the ACC and OFC.
“Overall, we found that OFC [signals] were more associated with real-world chronic pain, whereas ACC signals were more associated with acute, experimental pain,” said Shirvalkar.
More specifically, chronic pain states could be accurately decoded in all patients in the OFC alone. The chronic pain biomarker in the OFC was stable over the entire recording period, relating to pain intensity reliably over several months.
To measure biomarkers of acute pain, the authors time-locked brain recordings with experimental thermal pain stimuli applied to the patients’ body.
The team found that brain activity in the OFC and ACC could not be generalized in acute pain and chronic pain conditions, meaning that acute and chronic pain have distinct neural codes in these brain regions.
The team also found that data from the ACC, but not the OFC, could accurately predict acute pain in patients. Similarly, the OFC was more important than the ACC for decoding chronic pain signals, indicating different roles of the ACC and OFC in acute and chronic pain.
Specific biomarkers for specific chronic pain patients
One of the take-home messages of this study is that biomarkers for acute pain are very different from chronic pain.
“It shows that the spontaneous pain of a chronic pain patient is really not the acute pain you elicit with thermal stimulus,” said Geha.
This message leads to a central puzzle that arises from this study’s findings: How generalizable are these pain biomarkers to other types of chronic pain? As the recordings here were performed in patients with post-stroke pain and phantom limb pain, would recordings yield the same biomarkers for, as an example, chronic back pain or trigeminal neuralgia?
Geha thinks probably not. Instead, each chronic pain condition would likely have different pain biomarkers, owing to their different injury types and the different subjective experiences of chronic pain symptoms.
“[There are] only four subjects in this study, so it’s hard to generalize to all kinds of chronic pain conditions. This is an individual effect for one patient at a time measured over so many days. But this focus of the paper on sensitivity and specificity of recordings within individual patients was actually amazing” he said.
In other words, the chronic pain biomarkers in this study are more like fine needles into the brains of an individual patient than broader patterns of chronic pain in a larger population.
One of many chronic pain biomarkers?
This study is just the tip of the iceberg of information that can be mined from long-term LFP recordings in patients with chronic pain. There are different brain regions to be studied, different types of chronic pain, different pain drivers. It also opens up more questions about how the ACC and OFC are involved in acute and chronic pain, respectively. It’s an open book, and as Geha mentioned, an important reference paper to explain why people are exploring these regions in further studies.
Shirvalkar, also a neurologist, hopes the findings will someday have clinical relevance and is excited to explore this more in the future.
“It remains to be seen if these biomarkers are ‘causal,’ that is, if we can modify the biomarkers – will they help improve pain? If we interfere with the pain signals that we identified, can we short circuit and improve pain in individuals?” he said.
But a central open question from the study is whether the authors really found a true biomarker of pain. Are the electrodes recording the “pain generator” in the brain that drives chronic pain in these patients? Geha thinks it is unlikely.
“There might be an ‘evil demon’ somewhere in the brain that directly drives activity and matches chronic pain, but we cannot ‘see’ it here. What we are seeing is likely to be some kind of downstream phenomenon in the evaluation of pain experience,” Geha said.
Shirvalkar agreed, saying their findings are just “chapter one in the book of the holy grail of chronic pain.”
“It is almost certain pain signals exist across a widespread network involving many brain regions. As such, there are certainly other brain regions that harbor biomarkers,” he said.
Fred Schwaller, PhD, is a freelance writer based in Germany.
Image: Figure 2A. Example X-ray of a participant with bilateral implant of Activa PC + S DBS generators attached to depth leads in the ACC and paddle leads in the OFC (red highlights).