It’s only been 10 years since the emergence of the cancer neuroscience field. Back in 2013, landmark studies discovered that if peripheral neurons were ablated in a mouse model of prostate cancer, the cancerous cells were unable to develop (Magnon et al., 2013).
Since then, studies have suggested that nociceptors might be the new “bad guys” in cancer, helping to promote tumor growth in a number of different cancer types (Saloman et al., 2016). This work proposed that if you can stop nociceptor activity in a tumor, you can stop cancer progression.
Now, a new collaborative study led by Sebastien Talbot at Queen’s University in Canada has shown that nociceptors impair immunosurveillance in melanoma (cancerous melanocytes), thereby exacerbating skin cancer tumor growth. The study found that through the release of calcitonin gene-related peptide (CGRP), nociceptors exhaust immune T cells, thus limiting their ability to eliminate melanoma. Furthermore, silencing nociceptor activity decreased tumor growth and nearly tripled the survival rate of mice with skin cancer.
The study was published in Nature on November 2, 2022.
“This is a very important paper that brings together a lot of data about the role of sensory neurons in cancer. It ‘marries’ cancer neuroscience with immunotherapy and clinical elements that haven’t been recognized. This paper puts the focus on sensory neurons,” said Nicole Scheff, a cancer neuroscientist at the University of Pittsburgh, US, who was not a part of the study.
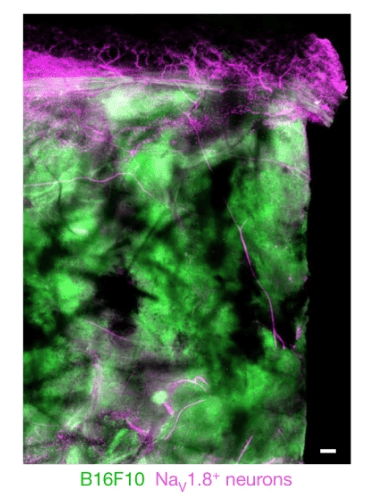
Nociceptors innervate skin cancer tumors
Senior author Sebastien Talbot explained that while cancer neuroscience is a relatively new field, the link between neurons and cancer has been known for a long time.
“It was actually shown way back in 1897 that neurons innervate skin tumors. In our study, we asked what roles these sensory neurons play in cancer, specifically melanoma,” Talbot told PRF.
Talbot’s team aimed to replicate these findings in skin cancer tumors. Initial experiments using biopsies from patients with melanoma found that these tumors indeed have increased innervation compared to adjacent healthy skin areas. What’s more, much of this innervation appeared to be from nociceptors, as TRPV1+ neuron innervation increased twofold in the tumor.
Nociceptors increase melanoma growth
Turning to animal models, the team next tested whether nociceptor activity affected melanoma growth. First, the authors used optogenetics to stimulate nociceptive neurons (expressing the sodium channel Nav1.8) that innervate melanoma in mice inoculated with B16F10 melanoma cells.
The team found that daily stimulation of nociceptors in the melanoma enhanced tumor growth, indicating a direct role of nociceptors.
In reciprocal experiments, they also ablated nociceptive (TRPV1+) DRG neurons in the same B16F10 mouse model of skin cancer. As expected, tumor growth was reduced, and these mice had a 2.5-fold increase in survival time.
Digging a little deeper, the team found that ablating nociceptors increased the immune response locally in the melanoma – with larger numbers of cytotoxic tumor-infiltrating CD8+ T cells. Further experimentation showed that silencing activated neurons with QX-314 – a sodium channel blocker – had the same effect of reducing both melanoma growth and T cell exhaustion.
“These findings show that nociceptor neurons somehow shape the function of the immune system in the tumor, thereby exacerbating cancer cell growth,” offered Talbot.
Releasing CGRP to suppress the immune system
To deepen their understanding, Talbot and his team investigated the interactions among nociceptors, melanoma cells, and immune T cells.
“It’s known that the sensory neurons play an important role in the progression of tumor cell growth by releasing factors that increase tumor growth. The question is what they are releasing, and how do they increase tumor growth,” said co-first author Maryam Ahmadi.
The authors turned back to the dish. Here they co-cultured DRG neurons with B16F10 cells and measured the release of a variety of pro-nociceptive peptides. They found that DRG neurons actively release CGRP in the presence of melanoma cells.
“But what was stimulating the neurons? We aimed to answer this question by creating a co-culture system designed to mimic the melanoma microenvironment, including immune T cells, naïve DRG neurons, and melanoma cancer cells,” said co-first author Mohammed Balood.
Here, melanoma cells were found to secrete SLPI (an antileukoproteinase) in the medium when in contact with DRG neurons. Further calcium imaging experiments found that SLPI activates sensory neurons, including capsaicin-sensitive nociceptors, and drives the release of CGRP.
Together, these experiments suggest a cycle of activation: Nociceptors innervate tumors in the skin and develop contacts with melanoma cells, which then secrete SLPI – in turn activating nociceptors and causing them to release CGRP.
Why isn’t melanoma painful?
“The mystery is that melanoma doesn’t have pain as a major driver. Many people do experience itch with skin cancer, but not always. How do we explain that? Honestly, we can’t yet,” said Balood.
The team found that SLPI injection into the hindpaw of naïve mice drives thermal hypersensitivity, showing the peptide has a direct role in pain hypersensitivity. The same was observed when B16F10 cells were injected into the hindpaw.
With all the SLPI and CGRP availability around skin cancer tumors, the expectation would be that patients experience hypersensitivity or allodynia around the area of tumor growth.
Scheff was also puzzled by the findings.
“Head and neck cancer is extremely painful, which makes sense when considering the afferent activity from the peripheral nervous system, but melanoma isn’t thought to be painful clinically. Is there a general release of an inhibitor factor in the tumor which stays local? Is it antidromic activation of nociceptors? It’s hard to say,” said Scheff.
CGRP exhaust T cells via RAMP1
The authors next wanted to explore how nociceptors were suppressing immune activity in these tumors via CGRP release.
Back at the dish, the team co-cultured immune cells and nociceptors, and tested whether nociceptors drive the expression of immune checkpoint receptors in CD8+ T cells. When activated, immune checkpoint receptors downregulate the ability of T cells to eradicate cancerous cells.
This concept has been utilized by the greater cancer research field to develop immune checkpoint inhibitors – in order to prevent T cell downregulation – thus reactivating the immune system so it can eliminate tumors.
The team found that stimulating nociceptors with capsaicin increased the expression of immune checkpoint receptors PD-1, LAG3, and TIM3. This was found to be mediated via CGRP-RAMP1 signaling (as RAMP1 is a CGRP receptor), and these effects were absent in Ramp1-/- mice.
These markers also suggested that T cells were “exhausted” by CGRP release and had limited cytotoxic potential. To test this further, the authors cultured B16F10 cells with cytotoxic T cells. Normally, the T cells would induce apoptosis of melanoma cells, but when stimulated with CGRP, the melanoma cells were not eliminated.
“CGRP is a known transmitter involved in immune responses, mediating neurogenic inflammation, particularly in the context of migraine. But these data showed that, in the presence of cancer, the role of CGRP seems to be different: Instead of activating an immune response, it suppresses it, which is already happening in cancer,” explained Scheff.
Reactivating the immune response to treat melanoma
When cancer grows, it begins to “shut down” the immune system. Talbot’s paper shows that nociceptors exacerbate this problem by also shutting down local T cell tumor infiltration. He explained how these findings open up some intriguing possibilities for new cancer treatments.
“In many cancers like pancreatic cancer, checkpoint inhibitor immunotherapies aren’t working well. Our data suggest that silencing nociceptors may help to release the block on T cells,” Talbot said.
To test this idea, the authors first looked at an immune checkpoint inhibitor called anti-PD-L1. Here, they administered anti-PD-L1 to mice who had been inoculated with melanoma, and then ablated TRPV1+ nociceptors.
They found that ablating nociceptors increased the ability of anti-PD-L1 to eliminate tumor cells. Moreover, it seemed directly linked to T cell unblocking, as T cell infiltration into the tumor was increased in mice with ablated nociceptors.
Finally, the team set to investigate whether blocking RAMP1 activity could reduce tumor growth. They applied the RAMP1 agonist in mice with melanoma, finding that these mice “succumb at a rate 2.6-fold lower” than mice with cancer and no RAMP1.
These findings provide early evidence that blocking CGRP release from neurons, or blocking RAMP1 on T cells, may help to reactivate local T cells and restart cancer immunosurveillance.
Clinical potential – almost, but not quite there
Of course, much work needs to be done before we see clinical treatments that modulate neuron-T cell interactions in melanoma, but Scheff explained that this research puts us on the right path.
“This work provides hope that CGRP antibodies can be given alongside anticancer drugs to make them more effective. In an ideal world, could we administer CGRP therapy two weeks prior to an immunotherapy agent to help reactivate the immune system and boost checkpoint inhibitor performance? This has to be worked out before moving into the clinic,” said Scheff.
The authors agreed that testing CGRP drugs like those used in migraine treatment is the obvious next step, but they also highlighted many open questions about the complex interactions among nociceptors, cancer types, and the wider immune system that first need to be addressed.
“For example, how do sensory neurons affect migration of other types of immune cells like dendritic cells to lymph nodes? The Sokol lab has already shown this is important for itch, so it might also be important for cancer,” said Balood (Perner et al., 2020).
Along with other cell types, Talbot’s team is also looking at different cancer types like breast cancer.
“What happens with other components of cancer tumors like fibroblasts, and in other types of cancers? It’s such an open field,” said Talbot.
Fred Schwaller, PhD, is a freelance science writer based in Germany.
Featured image: Balood et al., 2022. Figure 1A. Nociceptor (Nav1.8cre::tdTomatofl/WT; magenta) reporter mice were inoculated in the hindpaw with B16F10-eGFP cancer cells (i.d., 2 × 105 cells; green). Representative image of NaV1.8+ nerve fibres (magenta) innervating B16F10-eGFP-inoculated mouse skin after 22 days. Scale bar, 200 μm.